Xport-A functions as a chaperone by stabilizing the first 5 transmembrane domains of Rhodopsin-1
Por un escritor de hombre misterioso

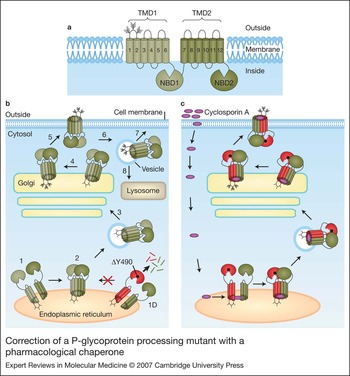
Chemical and pharmacological chaperones as new therapeutic agents, Expert Reviews in Molecular Medicine

Multiple TRP Regions Conferred XPORT Dependency (A) Percent amino acid

Structure network-based landscape of rhodopsin misfolding by mutations and algorithmic prediction of small chaperone action - Computational and Structural Biotechnology Journal

Xport-A functions as a chaperone by stabilizing the first five transmembrane domains of rhodopsin-1 - ScienceDirect

Xport-A functions as a chaperone by stabilizing the first five transmembrane domains of rhodopsin-1 - ScienceDirect

Xport-A functions as a chaperone by stabilizing the first five transmembrane domains of rhodopsin-1 - ScienceDirect

Xport-A functions as a chaperone by stabilizing the first 5 transmembrane domains of Rhodopsin-1
Rh1 rhodopsin mutants encode functional visual pigments.

Deletion of Emc1 in photoreceptor cells causes retinal degeneration in mice - Li - 2023 - The FEBS Journal - Wiley Online Library
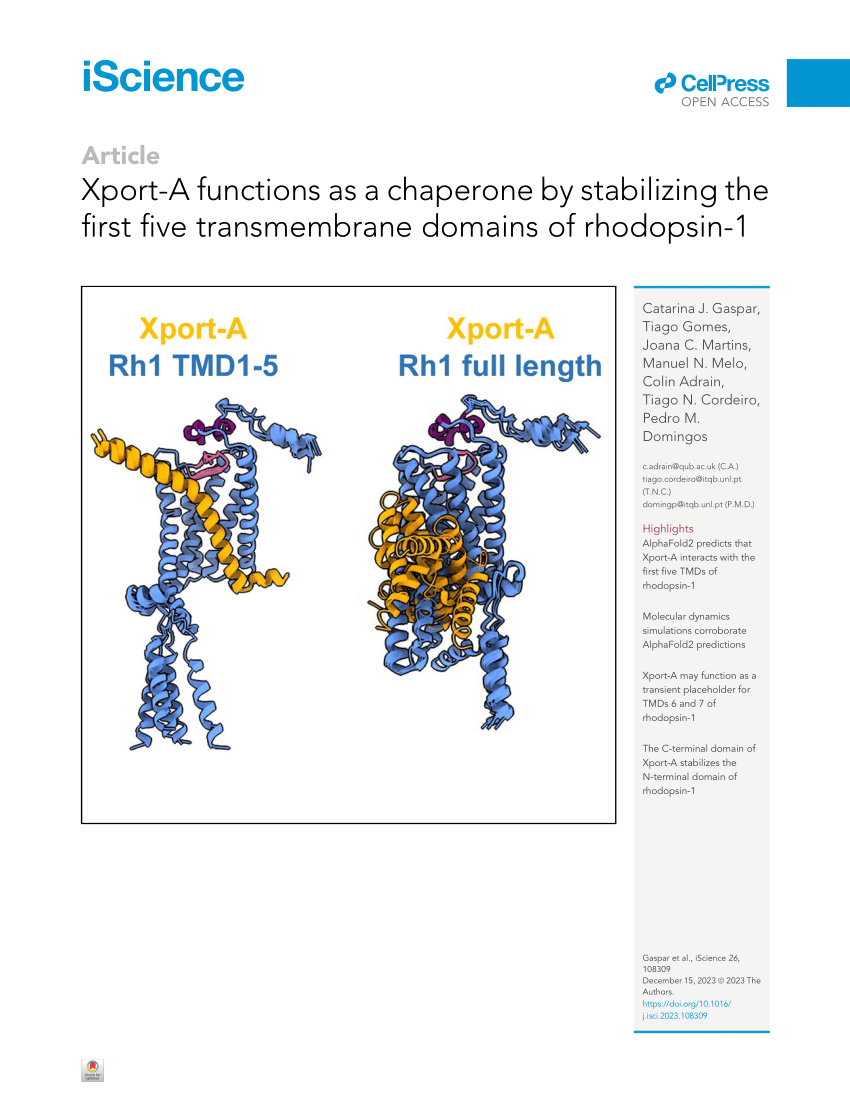
PDF) Xport-A functions as a chaperone by stabilizing the first five transmembrane domains of rhodopsin-1